Clinical Evaluation: The initial step in diagnosing Hirayama disease involves a thorough clinical assessment, focusing on the characteristic clinical features such as muscle weakness and atrophy, asymmetry of involvement, cold paresis, and preservation of sensory functions.
Electromyography (EMG) and Nerve Conduction Studies (NCS): EMG and NCS may be performed to evaluate the electrical activity of muscles and nerve conduction in the affected limbs. These tests can help exclude other neuromuscular disorders and confirm the diagnosis of a lower motor neuron pathology.
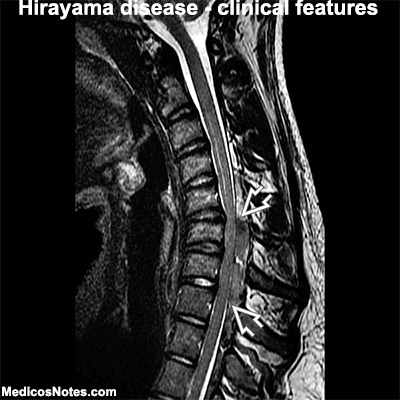
Magnetic Resonance Imaging (MRI): MRI is a key investigation in the diagnosis of Hirayama disease. The imaging findings are typically observed in the cervical spinal cord region, specifically the lower cervical segments (C5-C7). The following MRI findings are characteristic of Hirayama disease:
a. Forward Displacement of the Dural Sac: MRI often reveals a forward displacement of the dural sac during neck flexion. This occurs due to dynamic compression and elongation of the lower cervical spinal cord.
b. Posterior Epidural Space Enlargement: Enlargement of the posterior epidural space, located behind the dural sac, is a common finding in Hirayama disease. This can be visualized as a crescent-shaped area of CSF (cerebrospinal fluid) accumulation.
c. Flattening of the Spinal Cord: MRI may show flattening or concavity of the lower cervical spinal cord, particularly on the posterior aspect, during neck flexion. This is caused by compression of the spinal cord against the anterior vertebral column.
d. Vertebral Column Abnormalities: In some cases, MRI may reveal mild structural abnormalities in the vertebral column, such as focal kyphosis or loss of normal cervical lordosis. These abnormalities contribute to the spinal cord compression during neck flexion.
e. Normal Signal Intensity: Despite the clinical weakness and atrophy observed in Hirayama disease, MRI typically shows normal signal intensity of the affected spinal cord segments. This finding distinguishes it from other progressive motor neuron disorders characterized by abnormal signal intensity on MRI.
It is important to note that the MRI findings may be more pronounced during active neck flexion and may appear normal or less significant during neutral or extended neck positions. Thus, performing MRI with neck flexion is crucial to detect the characteristic findings associated with Hirayama disease.
While clinical evaluation and MRI findings are key in the diagnosis of Hirayama disease, additional investigations, such as laboratory tests and genetic studies, may be performed to exclude other conditions and assess for underlying genetic predispositions. The combination of clinical assessment and MRI findings is essential for a definitive diagnosis of Hirayama disease, enabling appropriate management and support for affected individuals.
Hirayama Disease - Investigation and MRI findings