Neuromyelitis optica (NMO), also known as Devic's disease, is a rare autoimmune disorder that affects the central nervous system. It is characterized by inflammation and damage to the optic nerves, which can cause vision loss, and the spinal cord, which can lead to weakness, numbness, and paralysis.
1. Basic pathology
The basic pathology of NMO involves the immune system attacking and damaging a protein called aquaporin-4, which is found in the cells that line the brain, spinal cord, and optic nerves. This damage causes inflammation and swelling, which can disrupt the normal functioning of these cells and lead to tissue damage.
2. Clinical presentations of NMOSD
The clinical presentation of Neuromyelitis Optica (NMO) can vary, but it typically involves inflammation and damage to the optic nerves and spinal cord. Some of the most common symptoms of NMO include:
1. Vision problems: NMO can cause inflammation and damage to the optic nerves, leading to vision loss or blindness in one or both eyes. This can be sudden and severe, and may be accompanied by eye pain or discomfort.
2. Weakness and numbness: Inflammation of the spinal cord can lead to weakness or paralysis in the limbs, as well as numbness or tingling sensations.
3. Difficulty with coordination: NMO can affect the cerebellum, which is the part of the brain that controls balance and coordination. This can lead to difficulty with walking, balance, and fine motor skills.
4. Bowel and bladder dysfunction: Damage to the spinal cord can affect the nerves that control the bladder and bowel, leading to problems with urination and defecation.
5. Pain: Some people with NMO may experience pain, particularly in the limbs or back.
The symptoms of NMO can occur suddenly and may worsen over time, although some people may experience periods of remission. NMO can be a progressive disease, and without treatment, it can lead to significant disability and loss of function. It is important to seek medical attention if you experience any of the symptoms associated with NMO.
3. Diagnostic tests of neuromyelitis optica
Diagnostic tests used in the evaluation of NMO may include:
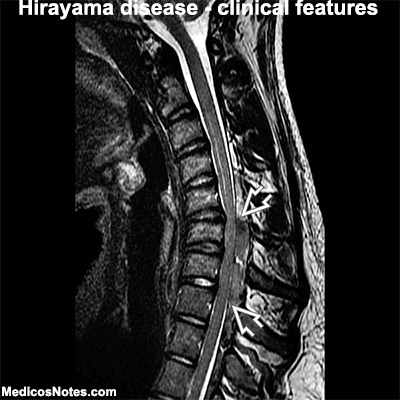
1. Magnetic resonance imaging (MRI): MRI can show the characteristic long segment lesions of the spinal cord, and the lesions of the optic nerves.
2. Blood tests: Blood tests can detect the presence of antibodies to AQP4, which are found in most cases of NMO.
3. Visual evoked potential (VEP) testing: This test measures the electrical activity of the visual pathway in response to visual stimuli, and can help identify abnormalities in the optic nerves.
4. Lumbar puncture: Lumbar puncture can be used to measure the levels of specific proteins in the cerebrospinal fluid, which may be elevated in NMO.
4.Treatment of NMO
The treatment of Neuromyelitis Optica (NMO) typically involves the use of immunosuppressive medications to reduce inflammation and prevent relapses. There are several medications that have been shown to be effective in treating NMO, including:
1. Glucocorticoids: These are a type of steroid medication that can reduce inflammation and swelling in the optic nerves and spinal cord. High-dose intravenous methylprednisolone is often used as the first-line treatment for acute NMO relapses.
2. Immunosuppressants: Medications that suppress the immune system can be used to prevent relapses of NMO. These include azathioprine, mycophenolate mofetil, and methotrexate. These medications are typically used as long-term maintenance therapy.
3. Plasma exchange: In cases of severe relapse, plasma exchange (also known as plasmapheresis) may be used to remove antibodies and other immune system proteins from the blood.
4. B-cell depleting therapy: Rituximab, a medication that depletes B-cells, has shown to be effective in reducing relapse rates in patients with NMO.
5. Newer treatments: There are newer medications like Eculizumab and Inebilizumab, which target the specific immune pathway responsible for causing NMO.
In addition to medications, supportive care such as physical therapy, occupational therapy, and speech therapy may be used to manage symptoms and improve quality of life. Regular follow-up with a neurologist or other specialist experienced in the management of NMO is important to monitor for relapses and adjust treatment as needed.
5.PROGNOSIS OF NMO
The prognosis of Neuromyelitis Optica (NMO) can vary depending on several factors, including the severity of symptoms, the frequency of relapses, and the age and overall health of the individual. NMO can be a progressive disease, and without treatment, it can lead to significant disability and loss of function.
However, with appropriate treatment and management, the prognosis for individuals with NMO has improved significantly in recent years. Newer medications and treatment strategies have been shown to be effective in reducing the frequency and severity of relapses, and many individuals with NMO are able to maintain a good quality of life.
It is important to note that NMO is a chronic condition, and individuals with NMO may experience periods of remission followed by relapses. Regular follow-up with a neurologist or other specialist experienced in the management of NMO is important to monitor for relapses and adjust treatment as needed.
Overall, the prognosis for individuals with NMO has improved in recent years with the development of newer treatments and management strategies. With appropriate care, many individuals with NMO are able to maintain good function and quality of life.






sound_medicos_notes.png)